How much heat is absorbed/released when 20.00 g of NH3(g) reacts in the presence of excess 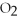 (g) to produce NO(g) and H2O(l) according to the following chemical equation? 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(l) ΔH° = 1168 kJ
(g) to produce NO(g) and H2O(l) according to the following chemical equation? 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(l) ΔH° = 1168 kJ
Definitions:
Therapeutic Factors
Are the elements in therapy or group therapy sessions that contribute to the healing and growth of participants, including empathy, insight, and support from others.
Group-Level Options
Choices or decisions made by a collective, reflecting the group's overall consensus or direction.
Therapeutic Group
A form of therapy where individuals gather in a group setting to discuss and work through personal issues with the guidance of a trained therapist.
Leadership Method
Approaches and strategies employed by leaders to guide, influence, and manage groups or organizations towards achieving goals.
Q11: How much heat is released when 95.0
Q21: The energy of motion is<br>A)electrical energy.<br>B)dam energy.<br>C)reserve
Q28: What is the hybridization of the oxygen
Q44: When heated,mercury(II)oxide decomposes into elemental mercury and
Q51: Based on formal charge considerations,the electron-dot structure
Q115: Iron crystallizes in a body-centered cubic cell
Q135: An element forms a body-centered cubic crystalline
Q143: The pressure in a container of gas
Q148: The hybrid orbital used by nitrogen to
Q157: The reaction 4 Ag(s)+ O<sub>2</sub>(g)→ 2 Ag<sub>2</sub>O(s)favors