When 5.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 61.16°C.If the specific heat of the solution is 4.18 J/(g ∙ °C) ,calculate 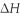 for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g)
for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g) 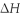 = ?
= ?
Definitions:
Advertising
A marketing strategy involving the use of paid communications to inform, persuade, and remind consumers about products, services, or brands.
Profits
The financial gain obtained when the revenue earned from business activities exceeds the expenses, costs, and taxes needed to sustain the activity.
Jeweler
A person or business that designs, makes, sells, or repairs jewelry.
Clothing Store
A retail establishment that sells garments and apparel for men, women, and children.
Q3: What is the geometry around the central
Q8: Assume that the vapor at point c
Q23: Although there are exceptions,which is most likely
Q26: A solution is prepared by dissolving 171
Q46: A solution is prepared by dissolving 171
Q48: At what temperature will sulfur hexafluoride molecules
Q71: Aqueous solutions of 30% (by weight)hydrogen peroxide,H<sub>2</sub>O<sub>2</sub>,are
Q126: Are the π-bonds in CH<sub>3</sub>CO<sub>2</sub><sup>-</sup> delocalized or
Q185: In the drawing of acetaldehyde,CH<sub>3</sub>CHO,the largest partial
Q193: Drawing (1)shows a system in which an