Acetylene torches utilize the following reaction: 2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g)
Use the given standard enthalpies of formation to calculate ΔH° for this reaction. 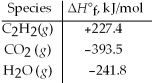
Definitions:
Confidence Interval
A segment of values, derived from analyzing a sample, that is likely to encapsulate the value of an undisclosed population attribute.
Sample Mean
The average of a subset of a population.
Margin of Error
A measure that indicates the range of values within which the true population parameter is expected to fall.
Sample Size
The number of observations or data points collected for a study or experiment.
Q24: Identify the packing in the figure shown
Q30: What are the bond angles in the
Q34: You have two samples of the same
Q58: Which salt has the lowest lattice energy?<br>A)picture
Q66: How many lone pairs are on the
Q111: A reaction is performed in a water
Q117: In the laboratory,hydrogen gas is usually made
Q135: A 0.529-g sample of gas occupies 125
Q142: The burning of sulfur-containing coal can lead
Q160: If the total pressure in the container