Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the pressure and number of moles of gas while keeping the temperature constant? 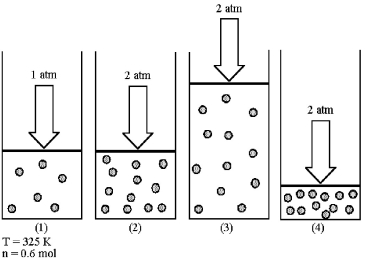
Definitions:
Q51: Which of the following should be nonplanar?
Q63: What is the expected freezing point of
Q64: How many liters of oxygen are needed
Q69: Use the following MO diagram for Be<sub>2</sub>,Be<sub>2</sub><sup>+</sup>,and
Q88: A 1.0 m aqueous BaI<sub>2</sub> solution will
Q104: How many anions are in the unit
Q120: The aquation of tris(1,10-phenanthroline)iron(II)in acid solution takes
Q125: Calculate Gibb's free energy and determine if
Q144: A gold ring is an example of
Q184: At STP how many liters of NH<sub>3</sub>