Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains 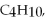 flask B contains SO2,and flask C contains He. Which flask contains the largest number of molecules?
flask B contains SO2,and flask C contains He. Which flask contains the largest number of molecules?
Definitions:
Personal-Document Approach
A method of studying personality that involves analysis of personal documents like diaries, letters, and autobiographies to understand individual lives and psychological patterns.
Self-Appraisal
The process of reflecting on, evaluating, and judging one's own performance, abilities, and qualities.
Human Nature
The fundamental qualities, such as thought processes, emotions, and behaviors, considered common among all human beings.
Becoming
The process of undergoing change or development towards a specific state or quality.
Q14: Over the time interval 300 to 400
Q23: If NO<sub>2</sub> and NH<sub>3</sub> are allowed to
Q37: If the Earth's ozone (O<sub>3</sub>)layer has a
Q54: Which law does the equation, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q59: Calculate the work energy,w,gained or lost by
Q62: According to the diagram,the solid phase of
Q87: To make a 0.125 m solution,one could
Q97: Imagine a reaction that results in a
Q111: How much heat (kJ)is required to convert
Q112: When 0.700 g of anthracene,C<sub>14</sub>H<sub>10</sub>,is combusted in