Effusion of a 1:1 mixture of two gases,represented by unshaded and shaded spheres in the diagram below,through a small pinhole produces the result shown below.The shaded spheres have a molecular mass of 20 amu.Which gas molecules have the higher average speed and what is the molecular mass of the unshaded molecules? 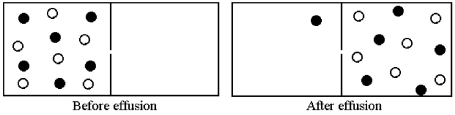
Definitions:
Social Approval
The positive recognition or acceptance from others in a social context, often influencing behavior and self-perception.
Peers
Those of similar status.
Socialization Agents
Individuals, groups, or institutions that play significant roles in integrating individuals into society by teaching societal norms, values, and practices.
Social Learning Theory
A theory that proposes that new behaviors can be acquired by observing and imitating others.
Q12: When 2.36 g of a nonvolatile solute
Q18: What is the mole fraction of oxygen
Q50: What is the pressure in a gas
Q53: For a particular process that is carried
Q55: At a given temperature the vapor pressures
Q66: Arrange in order from the smallest to
Q77: What is the geometry around the central
Q137: Identify the set of hybrid orbitals shown
Q181: Of XeF<sub>2 </sub>and XeF<sub>4,</sub>the one with the
Q191: Assuming that sea water is a 3.5