Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains 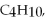 flask B contains SO2,and flask C contains He. Which flask contains the largest number of molecules?
flask B contains SO2,and flask C contains He. Which flask contains the largest number of molecules?
Definitions:
Vending Machine Products
Items sold through automatic retailing machines that require minimal human intervention, ranging from snacks and beverages to electronics and personal care items.
Significantly Higher Price
A pricing strategy or situation where goods or services are offered at prices considerably above the average market level, often reflecting superior quality or exclusivity.
Spreadsheet Simulation
A type of modeling that uses spreadsheet software to predict various outcomes based on different input variables, often used in finance and risk management.
Target Return
A financial goal for the return on an investment or business venture that a company or investor aims to achieve.
Q7: Ionic compounds consist of a single three-dimensional
Q11: How much heat is released when 95.0
Q15: The Lewis electron-dot structure of N<sub>2</sub> has
Q15: What is the volume of 10.0 g
Q40: What is the edge length of a
Q72: Which of the following liquids will exhibit
Q90: _ are used as a battery electrode
Q113: For the expansion of an ideal gas
Q114: For which reaction is H expected to
Q203: The HBr bond has a length of