The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 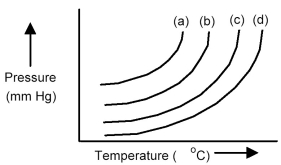
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for diethyl ether?
Definitions:
Advanced Placement
A program in the United States and Canada offering college-level curriculum and examinations to high school students.
International Baccalaureate
A globally recognized educational program that provides a challenging curriculum for students aimed at developing critical thinking and multicultural understanding.
Externally Scored Exams
Examinations that are marked or evaluated by individuals or entities outside of the educational institution where the exam was taken.
College Requirements
The set of academic and sometimes non-academic criteria that a student must fulfill to gain admission to a college or university.
Q2: The following drawing is a representation of
Q7: The sign of ΔG for a reaction
Q29: Given the following hypothetical reaction: 2 E(g)+
Q34: For the reaction,N<sub> </sub>H<sub>3</sub>(g)→ N(g)+ 3 H(g),one
Q43: Phosphorus pentachloride decomposes to phosphorus trichloride and
Q69: Which ion-dipole interaction results in the larger
Q109: Energy can be classified as either _
Q109: Lead has a radius of 154 pm
Q114: The solubility of argon in water at
Q122: What is the molecular geometry of SbCl<sub>3</sub>?<br>A)T-shaped<br>B)tetrahedral<br>C)trigonal