The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 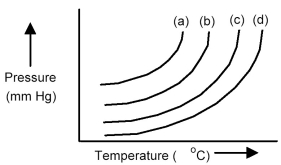
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for mercury?
Definitions:
Activity Analysis
The process of breaking down and examining activities within a process or system to improve efficiency and productivity.
Unit Fixed Costs
The consistent expenses incurred by a company for each unit produced that do not change with the volume of production.
Level of Activity
A measure of the volume of production or operations, often influencing costs and profitability in a business.
Variable Costs
Expenses that vary in relation to the amount of products or services a company generates.
Q25: A gas bottle contains 0.800 mol of
Q38: When solute is added to water,the solution
Q39: For the reaction,C<sub>2</sub>H<sub>2</sub>(g)→ 2 C(g)+ 2 H(g),one
Q66: How many Br<sup>-</sup> ions are around each
Q74: Chlorine reacts with chloroform according to the
Q92: A solution of LiCl in water is
Q101: The intermolecular forces formed when KI is
Q108: Which of the following is not a
Q116: Which of the following statements is false
Q187: A reaction is second order in NO