The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 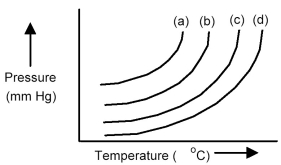
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for diethyl ether?
Definitions:
HRM Systems
Integrated methods and tools used to manage all aspects of the human resources function within an organization.
Global Managers
Executives or managers who are responsible for planning, directing, and coordinating operations in an international context.
Post-assignment Process
The procedures and activities that occur after the completion of a specific task or project, often involving evaluation and follow-up.
Cross-cultural Training
Educational programs designed to enhance individuals' understanding, skills, and competence in interacting effectively with people from different cultural backgrounds.
Q20: A steel bottle contains argon gas at
Q32: For the reaction shown below,what is the
Q39: The following set of data was obtained
Q42: The action of some commercial drain cleaners
Q70: Suppose you needed to closely monitor small
Q73: The oxidation of sulfur dioxide by oxygen
Q83: As a liquid evaporates at a temperature
Q87: Which type of bonding does Ca form
Q101: An unknown gas contains 83% C and
Q166: Which is a measure of the sum