The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 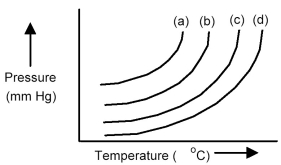
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for mercury?
Definitions:
Venture Growth
This describes the expansion and scaling process of a new business endeavor, focusing on increasing size, revenue, and market presence.
Entrepreneurial Leaders
Individuals who combine the vision and innovation of an entrepreneur with leadership skills to develop, organize, and manage a business.
Good Performance
The achievement of a set of standards or goals, often measured by productivity, efficiency, and quality, in any given task or job.
Share Of The Blame
The extent of responsibility that an individual or group holds in causing a particular negative outcome.
Q16: Which of the following pairs of solutions
Q25: A gas bottle contains 0.800 mol of
Q39: The following set of data was obtained
Q56: Which of the following exhibits ion-dipole forces?<br>A)KBr(s)<br>B)NaF(aq)<br>C)Ag(s)<br>D)Cl<sub>2</sub>(g)
Q82: How many grams of XeF<sub>6</sub> are required
Q115: In the reaction below,is energy released or
Q144: Which of the following does not affect
Q158: Which drawing above represents the system with
Q184: At STP how many liters of NH<sub>3</sub>
Q196: When 0.500 g of vitamin K is