Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 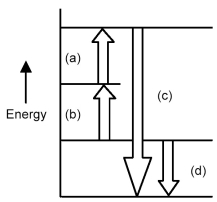
-Which arrow represents ΔHsoln?
Definitions:
America First Committee
An isolationist group that opposed American intervention in World War II prior to the attack on Pearl Harbor, advocating for the United States to prioritize domestic issues over international conflict.
Charles Lindbergh
An American aviator who became famous for making the first solo nonstop flight across the Atlantic Ocean in 1927, aboard the Spirit of St. Louis.
Lend Lease Program
A program initiated by the United States during World War II that allowed the U.S. to provide its allies with food, oil, and material without immediate payment in order to help defeat the Axis powers.
Battle of Britain
A major World War II air campaign fought primarily between the Royal Air Force of Great Britain and the Luftwaffe of Nazi Germany in 1940.
Q4: When baking soda is heated it decomposes
Q20: When 6.000 moles of H<sub>2</sub>(g)reacts with 3.000
Q38: Which of the following compounds forms an
Q65: The following reaction is second order in
Q109: K<sub>c</sub> = 1.2 × 10<sup>-42 </sup>at 500
Q120: Based on the relative strengths of the
Q131: The reaction below is second order in
Q135: Because the number of moles of gas
Q145: For which case would ΔH<sub>soln</sub> be expected
Q154: The half life of the reaction shown