A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 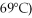 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 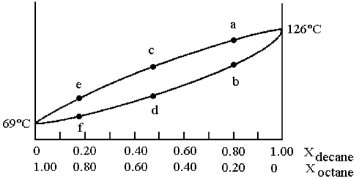
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,at what approximate temperature will the mixture begin to boil?
Definitions:
State Field
A component of a database or form that captures information related to the state or condition of an item or entity.
Property Box
A dialog box in software applications where users can view and modify settings and characteristics of selected elements or objects.
SQL Server
A relational database management system developed by Microsoft, designed to store, retrieve, and manage structured data.
Resources Needed Field
A specific area or field in a document or software where information about required resources is entered or displayed.
Q48: The cubic closest-packed arrangement of atoms is
Q49: What is the pressure (in mm Hg)of
Q60: A carbon dioxide monitoring product provides a
Q78: For which should the standard heat of
Q87: Which type of bonding does Ca form
Q91: The rate constant,k,for a first-order reaction is
Q108: What is the mole fraction of ethanol
Q109: Assume that you start with a mixture
Q114: Calculate the pH for an aqueous solution
Q165: What fraction of collisions will have sufficient