A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 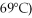 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 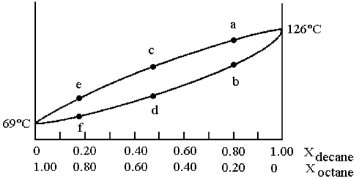
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,what is the liquid composition at the boiling point?
Definitions:
Negligible Fraction
A very small part or amount that is considered insignificant in size or importance.
Purely Competitive Market
A market structure featuring many sellers offering identical products, leading to competition based on price rather than product differentiation.
Demand Curve
A chart that displays the connection between a product's price and the amount consumers want to buy.
Nonprice Competition
A marketing strategy wherein companies focus on product or service differentiation rather than on changing prices.
Q9: The heat of vaporization of water at
Q13: Which type of spherical packing has the
Q53: Consider the first-order reaction A → B
Q55: In a flask containing 2.00 mol of
Q58: How much heat is released when 125.0
Q118: At 298 K which is larger rate
Q119: The approximate normal boiling point of this
Q126: Shown below is a concentration vs.time plot
Q139: A KCl solution is prepared by dissolving
Q146: The SI unit for energy is the