A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 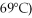 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 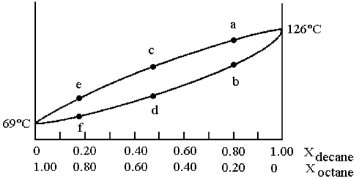
-Assume that the vapor at point c is condensed and reboiled.What is the liquid composition of the condensed vapor prior to reboiling?
Definitions:
Methanol
A simple alcohol with the formula CH3OH, known for being a volatile, colorless, and flammable liquid.
Base
A substance that can accept protons or release hydroxide ions in aqueous solutions.
pKa
A quantitative measurement that describes the acidity of a substance, specifically the negative log of the acid dissociation constant (Ka).
Acid Strength
A measure of the propensity of a compound to donate a proton (H+) in an aqueous solution, often quantified by the acid dissociation constant (Ka).
Q14: "Equal volumes of different gases at the
Q19: Which one of the following is not
Q46: A solution is prepared by dissolving 171
Q90: A steel bottle contains argon gas at
Q95: Which cation in each set would be
Q97: Of C<sub>2</sub>H<sub>5</sub>OH and C<sub>3</sub>H<sub>5</sub>(OH)<sub>3</sub> the one expected
Q117: _ properties depend only on the identity
Q118: At 298 K which is larger rate
Q174: For the first-order reaction,2 N<sub>2</sub>O(g)→ 2 N<sub>2</sub>(g)+
Q183: What is the hydronium ion concentration of