A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 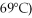 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 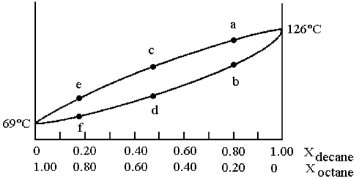
-Assume that the vapor at point c is condensed and reboiled.What is the boiling point?
Definitions:
Sodium Ions
Positively charged ions (Na+) essential for various physiological processes in the body, including nerve impulse transmission and muscle contraction.
Enzyme-Linked Receptors
Transmembrane proteins with a hormone-binding site outside the cell and an enzyme site inside the cell.
Tyrosine Kinase
An enzyme that phosphorylates the tyrosine part of proteins.
G Protein
A group of proteins that act as molecular switches across cell membranes, involved in transmitting signals from stimuli outside the cell.
Q55: For a reaction that follows the general
Q126: The reaction: 2 HI → H<sub>2</sub> +
Q130: What is the molality of ethanol in
Q133: The mixing of different gases by random
Q136: In a solution that is 75% ethyl
Q159: If one mole of each is dissolved
Q170: At a constant pressure of 2.50 atm
Q176: The rate-determining step in a reaction is
Q190: What is the conjugate acid of the
Q197: Chloroform has a boiling point of 61.1°C