Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 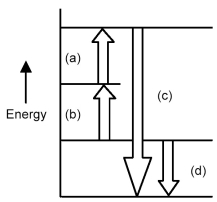
-Which arrows represent ΔHsolute-solute and ΔHsolvent-solvent?
Definitions:
AAA Corporate Bonds
High-grade bonds issued by corporations with the highest rating of creditworthiness, indicating very low risk of default.
Basis Points
A unit of measurement used in finance to describe the percentage change in value or rate, equal to one-hundredth of a percent or 0.01%.
Market Interest Rates
The rates of interest charged on loans or paid on deposits, determined by the demand and supply of credit in financial markets.
Municipal Bonds
Debt securities issued by municipalities to fund public projects like schools, highways, and infrastructure developments, offering tax-free interest income to investors.
Q28: The decomposition of dinitrogen pentoxide is described
Q32: For the isomerization reaction: butane ⇌ isobutane<br>K<sub>p</sub>
Q42: A slice of cheese pizza has a
Q46: For a reaction that follows the general
Q93: Hydroquinone,HOC<sub>6</sub>H<sub>6</sub>OH,can be formed by the reaction with
Q101: The decomposition of ozone in the stratosphere
Q102: The molecule believed to be most responsible
Q133: Which curve is the solvent and what
Q143: The pressure in a container of gas
Q163: The isomerization reaction,CH<sub>3</sub>NC → CH<sub>3</sub>CN,is first order