A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 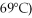 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 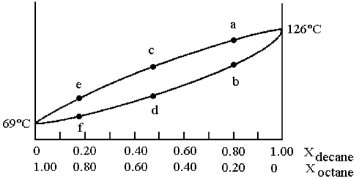
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,what is the vapor composition at the boiling point?
Definitions:
Central Vents
The main conduit in a volcanic system through which magma travels from beneath the Earth's surface to its exterior.
Flood Basalts
Large volcanic eruptions that cover large areas of the Earth's surface with basalt lava, forming thick lava flows.
Columbia Plateau
A geologic and geographic region in the Pacific Northwest of the United States characterized by a level area of basaltic lava flows.
Magma Volumes
The quantity of magma, which is molten rock beneath the Earth's surface.
Q26: The reaction for the decomposition of dinitrogen
Q39: Write the equilibrium equation for the forward
Q79: Assume that the vapor at point c
Q113: How many Br<sup>-</sup> ions are around each
Q125: An aqueous CsCl solution is 8.00 wt%
Q142: What is the rate law for this
Q163: The isomerization reaction,CH<sub>3</sub>NC → CH<sub>3</sub>CN,is first order
Q170: Hydrogen gas is collected over water in
Q174: How many grams of CO<sub> </sub> gas
Q188: What is the weight percent of vitamin