A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 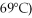 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 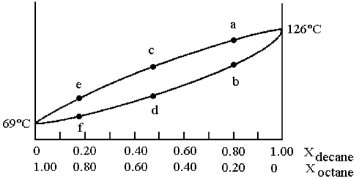
-Assume that the vapor at point c is condensed and reboiled.What is the boiling point?
Definitions:
Brain Function
The activities and processes that occur in the brain, enabling thought, perception, emotion, and behavior.
Body Project
The practice of actively shaping or modifying one's physical appearance through diet, exercise, fashion, or cosmetic surgery.
Societal Definitions
The meanings, norms, and values that a society or culture assigns to actions, characteristics, or phenomena.
Health And Beauty
The pursuit and industry focused on products and practices that enhance physical well-being and aesthetic appeal.
Q36: Which equation best represents the reaction?<br>A)4A(g)→ B(g)+
Q60: The normal boiling point for HBr is
Q80: Which transition could occur if a solid
Q135: An element forms a body-centered cubic crystalline
Q139: The following set of data was obtained
Q148: How much heat is transferred per 5.00
Q148: At STP if 1.00 mole of gas
Q149: "If a stress is applied to a
Q149: What is the temperature of SO<sub>2</sub> gas
Q167: One mole of gas at 25°C in