Methanol can be produced by the following reaction: CO(g) + 2 H2(g) → CH3OH(g) .
How is the rate of disappearance of hydrogen gas related to the rate of appearance of methanol?
- 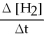 = ?
= ?
Definitions:
Units
In economics, units refer to the quantifiable measures used to express the quantity of an item, such as pieces, kilograms, liters, etc., used in transactions or production.
Total Cost
The complete cost of production, encompassing both fixed and variable costs, for a good or service.
Average Fixed Cost
The fixed costs of production divided by the quantity of output produced; decreases as production increases.
Average Variable Cost
The per unit cost of variable inputs divided by the total quantity of output produced, reflecting the variable cost of production.
Q3: The following reaction is first order in
Q9: The normal boiling point of pure benzene
Q38: Which of the following compounds forms an
Q56: What phase changes occur when the pressure
Q89: What is the empirical formula of the
Q115: What is the pH of a solution
Q120: Potassium hydrogen phthalate (molar mass = 204.2
Q126: The reaction: 2 HI → H<sub>2</sub> +
Q127: The equilibrium constant is equal to 5.00
Q144: A gold ring is an example of