The reaction for the decomposition of dinitrogen monoxide gas to form oxygen radicals is: 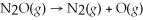 .If the rate constant is 3.04 × 10-2 s-1 and the frequency factor is 8.00 × 1011 s-1,what is the activation energy for the first-order reaction at 700°C?
.If the rate constant is 3.04 × 10-2 s-1 and the frequency factor is 8.00 × 1011 s-1,what is the activation energy for the first-order reaction at 700°C?
Definitions:
Fifth Disease
A mild viral infection that typically causes a bright red rash on the cheeks, often prevalent among children.
Herpetic Gingivostomatitis (HGS)
A viral infection affecting the mouth and gums, marked by sores, fever, and swollen gums, commonly caused by the herpes simplex virus.
Topical Anesthetics
Medications applied directly to the skin or mucous membranes to temporarily numb the area, providing pain relief for procedures or conditions causing discomfort.
Acyclovir (Zovirax)
An antiviral drug used to treat infections caused by herpes viruses, including genital herpes, cold sores, shingles, and chickenpox.
Q7: Molality is defined as moles of solute
Q8: What are the Br∅nsted-Lowry acids in the
Q14: What volume of a 0.716 M KBr
Q27: The first-order isomerization reaction: cyclopropane → propene,has
Q69: Which of the areas designated A,B,and C
Q132: At 1000 K,K<sub>p</sub> = 19.9 for the
Q143: The pressure in a container of gas
Q148: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q149: "If a stress is applied to a
Q195: A solution with a hydroxide ion concentration