Consider the first-order decomposition of A molecules (shaded spheres) in two vessels of equal volume.What is the half-life of decomposition in vessel (b) relative to the half-life of decomposition in vessel (a) ? 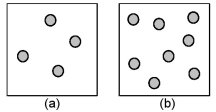
Definitions:
Geert Hofstede
A Dutch social psychologist known for his research on the comparisons of cultures across nations, leading to the development of his cultural dimensions theory.
Human Resources Management (HRM)
The strategic approach to the effective management of people in an organization, ensuring their contribution towards the achievement of the organization's goals.
Geocentric Company
A business that adopts a global approach in its operations and personnel practices, ignoring national boundaries.
Domestic Perspective
Views or analysis coming from or related to one's own country or the internal affairs of a company.
Q6: The approximate normal melting point of this
Q20: How many grams of pyridine are there
Q38: Which of the following compounds forms an
Q46: A solution is prepared by dissolving 171
Q84: Which of the following statements is true
Q108: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q141: Which one of the following salts,when dissolved
Q170: If the units for rate are M
Q187: What is the strongest acid among the
Q216: One way to prepare a solution with