Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 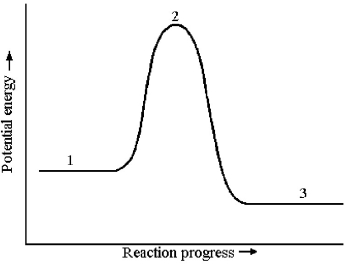
-The activation energy for the forward reaction is given by the difference in energy between which two reaction stages?
Definitions:
Sympathetic Chain
A part of the autonomic nervous system consisting of a series of ganglia that run alongside the spinal column and influence internal organs by releasing neurotransmitters.
Collateral Ganglia
Sympathetic ganglia at the origin of large abdominal arteries; include the celiac, superior, and inferior mesenteric arteries; also called prevertebral ganglia.
Preganglionic Fibers
Nerve fibers that run from the central nervous system to a ganglion in the autonomic nervous system.
Thoracic
Relating to the thorax, the area of the body between the neck and the abdomen that contains the heart and lungs.
Q21: At an elevated temperature,K<sub>p</sub> = 4.2 ×
Q120: Which of the following solutions will have
Q122: Oxalic acid can donate two protons to
Q123: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q147: Which of the elementary reactions shown above
Q169: Which of the following can be classified
Q170: If the units for rate are M
Q170: Molarity is defined as _,whereas molality is
Q178: A three-step mechanism has been suggested for
Q227: Determine the ammonia concentration of an aqueous