Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 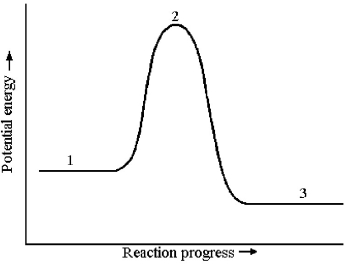
-The energy of reaction,ΔE,is given by the difference in energy between which two reaction stages?
Definitions:
Subcultures
Cultural groups within a larger culture, often with beliefs or interests at variance with those of the larger culture.
Symbols
Objects, signs, or emblems that represent or stand for something else, often communicating complex ideas, concepts, or values.
Ethnic Group
A community of people who share a common cultural background, ancestry, language, history, and often, geographic origin.
Sexual Behavior
The manner in which individuals express their sexuality, encompassing activities, practices, and psychological aspects associated with sexuality.
Q6: What is the species present at reaction
Q66: When dissolved in water,which of the following
Q92: A solution of LiCl in water is
Q111: How much heat (kJ)is required to convert
Q141: The first-order reaction,SO<sub>2</sub>Cl<sub>2</sub> → SO<sub>2</sub> + Cl<sub>2</sub>,has
Q160: Commercial cold packs often contain solid NH<sub>4</sub>NO<sub>3</sub>
Q171: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q177: At 50°C the value of K<sub>w</sub> is
Q185: What is the hydronium ion concentration and
Q221: Dihydrogen phosphate H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,has an acid dissociation constant