Methanol can be produced by the following reaction: CO(g) + 2 H2(g) → CH3OH(g) .
How is the rate of disappearance of hydrogen gas related to the rate of appearance of methanol?
- 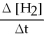 = ?
= ?
Definitions:
Fermentation System
A biological process using microorganisms to convert organic materials into alcohol, gases, or acidic products.
Entrepreneur
An individual who creates a new business, bearing most of the risks and enjoying most of the rewards, often seen as an innovator and a source of new ideas, goods, services, and business/or procedures.
Production Possibility Frontier
A graph showing the highest possible combinations of two goods that can be made using existing resources and technology.
Output Efficiency
The optimized state where goods or services are produced at the lowest possible cost and with the best allocation of resources.
Q12: For the reaction shown below the value
Q98: At 25°C the vapor pressures of benzene
Q101: The solubility of a gas in a
Q103: The solubility of gaseous solutes in liquid
Q114: The following set of data was obtained
Q124: An aqueous reaction occurs by a two-step
Q130: Which of the following forms a molecular
Q143: The rate constant,k,for a first-order reaction is
Q156: The aquation of tris(1,10-phenanthroline)iron(II)in acid solution takes
Q176: Which one of the following species acts