Consider the first-order decomposition of A molecules (shaded spheres) in two vessels of equal volume.What is the half-life of decomposition in vessel (b) relative to the half-life of decomposition in vessel (a) ? 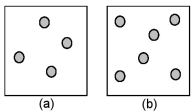
Definitions:
Enthalpy Reaction
A thermodynamic quantity equivalent to the total heat content of a system, representing the sum of its internal energy and the product of its pressure and volume, especially useful in the study of heat exchange in chemical reactions.
Electrochemical Reaction
A chemical reaction that involves the transfer of electrons from one species to another, often associated with electricity.
Exergonic
Describing a chemical reaction that releases energy to its surroundings, typically in the form of heat or work.
Endergonic
Endergonic refers to a chemical reaction where energy is absorbed from the surroundings, generally requiring an input of energy to proceed.
Q10: The rubbing alcohol sold in drug stores
Q24: Identify the packing in the figure shown
Q25: Which of the following solutions has the
Q53: Barium has a radius of 224 pm
Q66: If 0.40 mol of NaN<sub>3 </sub>reacts completely
Q95: A mechanism for a naturally occurring reaction
Q115: A solution is 2.25% by weight NaHCO<sub>3</sub>.How
Q136: What is the equilibrium constant expression (K<sub>a</sub>)for
Q146: A 1.25 × 10<sup>-4</sup> M solution of
Q169: Which of the following can be classified