Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 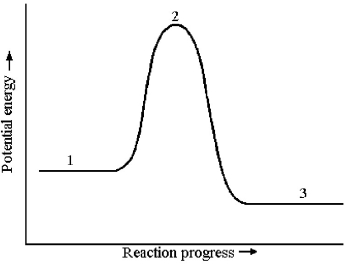
-What is the species present at reaction stage 3?
Definitions:
Electron Configuration
The positioning of electrons in the atomic or molecular orbitals pertaining to an atom or a molecule.
Arsenic
A chemical element with symbol As, known for its various allotropes and toxic compounds, used in semiconductors, pesticides, and historically as a poison.
Electron Configuration
The distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
Nitrogen
A colorless, odorless, tasteless gas that constitutes 78% of the Earth's atmosphere; it is a nonmetallic element with symbol N.
Q36: Which equation best represents the reaction?<br>A)4A(g)→ B(g)+
Q43: Phosphorus pentachloride decomposes to phosphorus trichloride and
Q45: To make a 3.00 m solution,one could
Q51: The following pictures represent mixtures of cis-C<sub>2</sub>H<sub>2</sub>X<sub>2</sub>
Q59: The decomposition of cyclopropane,was observed at 500°C
Q80: Hydrogen peroxide decomposes to water and oxygen
Q106: A mechanism for a naturally occurring reaction
Q129: If 1.0 gram each of Cl<sub>2</sub>,CO<sub>2</sub>,N<sub>2</sub>,and O<sub>2</sub>
Q156: Calculate the pH of a 0.060 M
Q157: At 25°C,the pH of a vinegar solution