Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 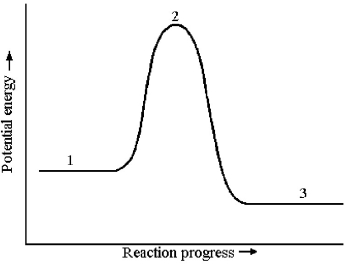
-The activation energy for the forward reaction is given by the difference in energy between which two reaction stages?
Definitions:
Methodologies
refers to the systematic, theoretical analysis of the methods applied to a field of study, encompassing the principles behind the methods employed in research.
Psychophysiological Disorder
Health problems that occur due to the interaction of psychological, behavioral, and physiological factors.
Ulcer
A lesion that forms in the wall of the stomach or of the duodenum.
Hypotension
A medical condition characterized by blood pressure that is too low, which can lead to symptoms like dizziness or fainting.
Q2: When a particular solid begins to dissolve
Q11: What is the mole fraction of ethanol
Q18: For the reaction shown below,if the rate
Q40: What is the edge length of a
Q44: A three-step mechanism has been suggested for
Q82: KBr crystallizes in a cubic unit cell
Q109: Lead has a radius of 154 pm
Q134: Consider the first-order decomposition of A molecules
Q142: What is the rate law for this
Q156: Which picture (2)-(4)represents the equilibrium mixture at