Consider a reaction that occurs by the following one-step mechanism:
A2 + B2 → 2 AB
The potential energy profile for this reaction is shown below. 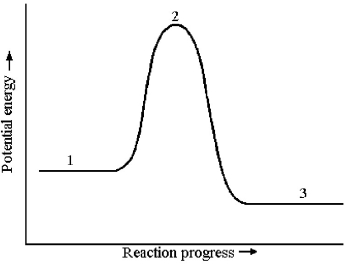
-The energy of reaction,ΔE,is given by the difference in energy between which two reaction stages?
Definitions:
Work-Related Situation
Refers to any circumstance, task, or event that occurs in the professional or work setting, impacting the behavior and performance of individuals or groups.
Mobilization Strategies
Methods or plans designed to assemble, organize, and deploy resources or people towards achieving specific goals, especially in social or political contexts.
Capacity-building Strategies
Approaches or methods aimed at developing skills, abilities, and competencies in an individual, organization, or community to increase performance and achieve goals.
Social Action Strategies
Plans of action designed to address social issues and affect societal change.
Q3: The dissolution of calcium hydroxide is exothermic:
Q39: Identify the Br∅nsted-Lowry bases.<br>A)(1)and (3)<br>B)(1)and (4)<br>C)(2)and (3)<br>D)(2)and
Q60: A 0.50 m solution of which solute
Q71: A supercritical fluid refers to a substance<br>A)above
Q97: The first-order reaction,SO<sub>2</sub>Cl<sub>2</sub> → SO<sub>2</sub> + Cl<sub>2</sub>,has
Q120: The aquation of tris(1,10-phenanthroline)iron(II)in acid solution takes
Q137: If K<sub>c</sub> equals 0.110 at 25°C for
Q147: Which of the elementary reactions shown above
Q156: Which drawing above represents the system with
Q183: What is the hydronium ion concentration of