Consider a reaction that occurs by the following mechanism:
A + BC → AC + B
AC + D → A + CD
The potential energy profile for this reaction is shown below. 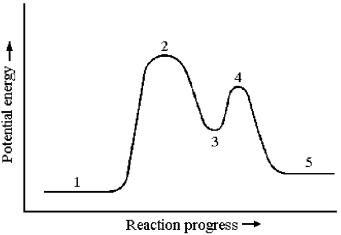
-Intermediates occur at which reaction stages?
Definitions:
Compiled Language
A programming language whose code must be translated (compiled) into executable machine code before it can be run.
Executable Program
A file containing code that a computer's operating system can directly execute.
Q19: How many grams of KBr are required
Q20: The esterification of acetic acid and ethanol
Q33: What is the pH of a 0.020
Q39: The following set of data was obtained
Q48: Two beakers,one with pure water (light gray)and
Q84: Which of the following statements is true
Q86: If one mole of gas occupies 22.4
Q93: Which curve is the solvent and what
Q166: The vapor pressure of water at 25°C
Q222: What is the pH of a solution