Find the equilibrium constant for the reaction: A(g) + B(g) ⇌ 2C(g) at 25°C when k equals 1.4 × 10-12 M-1s-1 for the reaction A(g) + B(g) → 2C(g) at 25°C and k equals 2.7 × 10-13 M-1s-1 for the reaction: 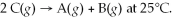
Definitions:
Efficient Market
A financial market theory suggesting that asset prices fully reflect all available information, making it impossible to consistently achieve higher than average returns.
Insider Trading Laws
Regulations that prohibit the trading of a company's stocks or other securities by individuals with access to non-public, material information about the company.
Market Efficiency
The degree to which stock prices and other securities prices fully reflect all available information and are accurate reflections of the true value of investments.
Capital Markets
Markets that deal in the buying and selling of long-term debt or equity-backed securities to help in capital acquisition.
Q24: Which nonequilibrium mixture will react in the
Q41: The following reaction is second order in
Q44: If K<sub>c</sub> is the equilibrium constant for
Q64: Which one of the following salts,when dissolved
Q78: The dose of amoxicillin given to a
Q87: What is the pH of a 0.020
Q107: The concentration of H<sub>3</sub>O<sup>+</sup> in human sweat
Q131: Sodium hypochlorite,NaOCl,is the active ingredient in household
Q164: Hydrogen iodide decomposes at 800 K via
Q213: Which one of the following is not