Shown below is a concentration vs.time plot for the reaction A ⇌ B.For this reaction the value of the equilibrium constant is 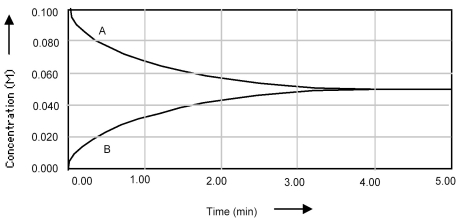
Definitions:
Electrons
Subatomic particles with a negative charge, found orbiting the nucleus of an atom, involved in chemical bonds and electrical conductivity.
Enthalpy
A thermodynamic quantity equivalent to the total heat content of a system, representing the amount of energy needed to create a system plus the work needed to make room for it in its environment.
Free Energy
A thermodynamic quantity that represents the amount of work that can be performed by a system at a constant temperature and pressure.
Entropy
Disorderliness; a quantitative measure of the amount of the random, disordered energy that is unavailable to do work.
Q10: The rubbing alcohol sold in drug stores
Q51: Arrange the following compounds in order of
Q76: At some temperature,a 4.0 L flask is
Q102: The equilibrium constant,K<sub>p</sub>,equals 3.40 for the isomerization
Q106: What is the pH of a solution
Q124: What is the molar solubility of AgCl
Q133: Which curve is the solvent and what
Q147: Which of the elementary reactions shown above
Q160: The following reaction is first order,C<sub>2</sub>H<sub>6</sub> →
Q171: What volume of 3.00 M CH<sub>3</sub>OH solution