The following picture represents the equilibrium state for the reaction A2 + B2 ⇌ 2AB.What is the relationship between the rate constant for the forward reaction,kf,and the rate constant for the reverse reaction kr? 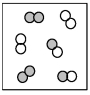
Definitions:
Heavy Smoker
An individual who smokes tobacco products excessively, significantly increasing their risk of developing health problems.
Caffeine
A stimulant found in coffee, tea, chocolate, and some medications, known for its ability to enhance alertness and reduce feelings of fatigue.
Cardiovascular Development
The process of growth and maturation of the heart and blood vessels throughout a person's life.
Without Defects
Describes a condition or object that is free from flaws, imperfections, or deficiencies.
Q85: For the reaction shown below,the rate of
Q90: What is the pH of the resulting
Q91: The rate constant,k,for a first-order reaction is
Q98: Addition of 0.0125 mol KOH to 150
Q102: What is the approximate pH at the
Q112: If an equal number of moles of
Q121: At a certain temperature,hydrogen and iodine react
Q129: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q144: The solubility of 1:1 salts is measured
Q166: What is the value for K<sub>c</sub> for