Multiple Choice
The following picture represents the equilibrium state for the reaction A2 + B2 ⇌ 2AB.What is the relationship between the rate constant for the forward reaction,kf,and the rate constant for the reverse reaction kr? 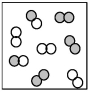
Definitions:
Related Questions
Q11: Which of the elementary reactions shown above
Q36: What are the conjugate acid-base pairs in
Q62: A solution is prepared by dissolving 17.75
Q81: Cerium(IV)ion reacts with thallium(I)ion in a one-step
Q110: In which case should CO<sub>2</sub>(g)be more soluble
Q123: 0.10 M potassium chromate is slowly added
Q148: For a liquid solution made by dissolving
Q154: Using the conjugate acid-base pairs listed below,complete
Q166: What is the pH at the first
Q167: Nitrogen dioxide decomposes at 300°C via a