Multiple Choice
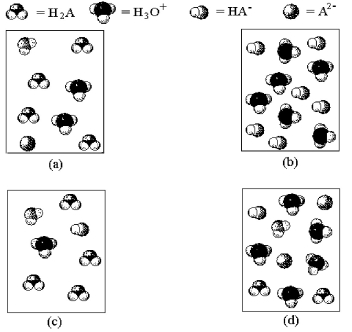
-Which of the above pictures represents a solution of a weak diprotic acid H2A for which Ka1 >> Ka2? (Water molecules have been omitted for clarity. )
Definitions:
Related Questions
Q3: A reaction has ΔG° = + 21.5
Q30: The pH of 0.255 M HCN is
Q36: What are the conjugate acid-base pairs in
Q36: The balanced equation for the solubility equilibrium
Q43: What is the geometric shape of the
Q95: Which provides the greatest increase in entropy?<br>A)H<sub>2</sub>O
Q100: Which is the best acid to use
Q117: Water can be made from elemental hydrogen
Q164: What is the [CH<sub>3</sub>CO<sub>2</sub><sup>-</sup>]/[CH<sub>3</sub>CO<sub>2</sub>H] ratio necessary to
Q230: What is the pH of a 0.10