Multiple Choice
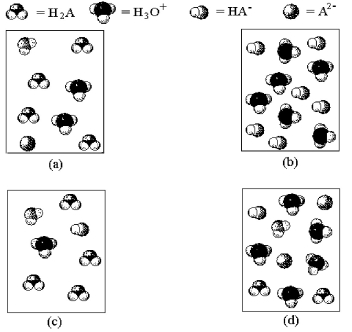
-Which of the above pictures represents a solution of a diprotic acid H2A for which Ka1 = ∞ and Ka2 is exceptionally small.(Water molecules have been omitted for clarity. )
Definitions:
Related Questions
Q10: What is the pH at the equivalence
Q19: What is the equation relating the equilibrium
Q52: Calcium carbonate is relatively insoluble and the
Q55: For the process CaCO<sub>3</sub>(calcite)→ CaCO<sub>3</sub>(aragonite)ΔH° = -0.21
Q62: Phenobarbital has a pK<sub>a</sub> = 7.4.Compared to
Q84: At 2600 K,ΔG° = 775 kJ for
Q124: The figure represents the spontaneous evaporation of
Q134: Bromothymol blue indicator changes color from yellow
Q170: In a galvanic cell,the half-reaction H<sub>2</sub>(g)+ 2
Q183: Which picture represents the equilibrium state of