Multiple Choice
Calculate the pH for an aqueous solution of pyridine that contains 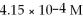 hydroxide ion.
hydroxide ion.
Definitions:
Related Questions
Q38: For which of the following will the
Q48: What is the identity of M in
Q55: For the isomerization reaction: butane ⇌ isobutane<br>K<sub>p</sub>
Q84: Write the overall cell reaction for the
Q99: The equivalence point pH of the titration
Q106: What is the pH of a solution
Q116: Which acid has the smallest value of
Q133: What is the equilibrium equation for the
Q145: Calculate the concentration of bicarbonate ion,HCO<sub>3</sub><sup>-</sup>,in a
Q168: A reaction has the rate law Rate