What is the hydronium ion concentration of a 0.200 M hypochlorous acid solution with 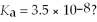 The equation for the dissociation of hypochlorous acid is: HOCl(aq) + H2O(l) ⇌ H3O+(aq) + OCl-(aq) .
The equation for the dissociation of hypochlorous acid is: HOCl(aq) + H2O(l) ⇌ H3O+(aq) + OCl-(aq) .
Definitions:
Labor Shortages
A situation in which employers have difficulty filling positions with qualified candidates, often due to a lack of available or skilled workers.
Short-Run Aggregate Supply
The aggregate volume of products and services that companies intend to sell within a brief period in the economy, based on existing price levels.
Long-Run Aggregate Supply
Represents the total production of goods and services in an economy at full employment, without any market frictions.
Aggregate Quantity Demanded
The total amount of a good or service that consumers are willing and able to purchase at a given price level in an economy.
Q18: The Boltzmann formula is S = k
Q57: Which has the lowest entropy?<br>A)CH<sub>3</sub>OH(s,-26°C)<br>B)CH<sub>3</sub>OH(s,-13°C)<br>C)CH<sub>3</sub>OH(l,16°C)<br>D)CH<sub>3</sub>OH(l,30°C)
Q77: The equilibrium constant,K<sub>p</sub>,equals 3.40 for the isomerization
Q97: Erythromycin is a basic antimicrobial with pK<sub>b</sub>
Q101: For the reaction N<sub>2</sub>(g)+2 O<sub>2</sub>(g)→ 2 NO<sub>2</sub>(g),ΔH°
Q104: What is the best balanced chemical equation
Q124: The figure represents the spontaneous evaporation of
Q132: At 1000 K,K<sub>p</sub> = 19.9 for the
Q150: From the following chemical reactions determine the
Q158: Which is a net ionic equation for