The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 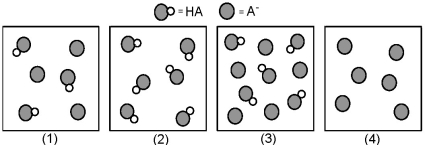
-Which solution has the largest percent dissociation of HA?
Definitions:
Stage 1 Sleep
The initial phase of sleep, characterized by light sleep from which one can be easily awakened, and where the eyes move slowly and muscle activity slows.
Aldehyde Dehydrogenase
An enzyme that plays a crucial role in metabolizing alcohols in the body, helping to convert aldehydes into acids.
Chemical
A substance produced by or used in a chemical process, often characterized by a specific molecular composition.
Mindfulness Meditation (MM)
A form of meditation that provides clients with techniques they can use to focus on the present moment rather than ruminate about problems.
Q3: The dissolution of calcium hydroxide is exothermic:
Q22: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q34: What is the equilibrium equation for the
Q41: Under which of the following conditions would
Q51: For initial state 1 what is the
Q60: Which of the following salts are acidic?<br>A)LiCl,NaCl,KCl<br>B)NH<sub>4</sub>Cl,CuCl<sub>2</sub>,AlCl<sub>3</sub><br>C)NaCH<sub>3</sub>CO<sub>2</sub>,LiCH<sub>3</sub>CO<sub>2</sub>,RbCH<sub>3</sub>CO<sub>2</sub><br>D)KCl,NH<sub>4</sub>Cl,Na<sub>2</sub>CO<sub>3</sub>
Q87: A fuel cell is a galvanic cell
Q89: What is the relationship between ΔG and
Q100: What are the coefficients in front of
Q123: Calculate the pH of a 0.20 M