The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity. ) 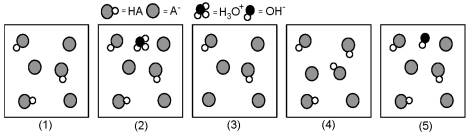
-Which picture represents the equilibrium state of the solution after addition of one OH- ion to the solution shown in picture (1) ?
Definitions:
Simple Linear Regression
A statistical method used to model the relationship between a dependent variable and a single independent variable, assuming a linear relationship.
Residual
The difference between the observed value and the value predicted by a model, indicating the error in prediction for that specific point.
Least-squares Line
A line of best fit determined by the least squares method, which minimizes the sum of squared vertical distances between the actual data points and the predicted points on the line.
Unbiased Estimator
A statistical estimator whose expected value exactly equals the parameter it estimates.
Q34: What is the strongest acid among the
Q38: Calculate the pH of a 0.100 M
Q42: A 0.50 M solution of a weak
Q45: For which of these solutions is pH
Q65: What is the equilibrium constant expression for
Q96: What is the sign of ΔS for
Q119: An aqueous reaction occurs by a two-step
Q130: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q141: At a certain temperature,K<sub>c</sub> equals 1.4 ×
Q183: What is the hydronium ion concentration of