The following pictures represent solutions at various points in the titration of a weak acid HA with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 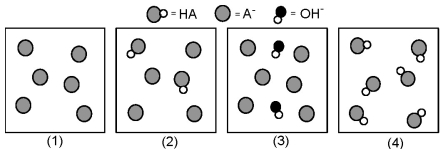
-Which picture represents the solution at the equivalence point?
Definitions:
Labor Force
The total number of people who are employed or actively looking for employment.
Manufacturing Economy
An economy that is primarily based on the production and export of manufactured goods.
Union Organizing
The process by which workers and labor unions work together to form or join a union in order to negotiate collectively with their employers regarding wages, hours, benefits, and working conditions.
Work Time
The duration or period during which an employee is considered to be on duty or performing activities related to their job.
Q53: In addition to a beta particle,what is
Q57: Which has the lowest entropy?<br>A)CH<sub>3</sub>OH(s,-26°C)<br>B)CH<sub>3</sub>OH(s,-13°C)<br>C)CH<sub>3</sub>OH(l,16°C)<br>D)CH<sub>3</sub>OH(l,30°C)
Q69: Oxalic acid,H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> has acid dissociation constants K<sub>a1</sub>
Q77: In figure (1)below argon atoms,represented by unshaded
Q79: Which statement about buffers is true?<br>A)Buffers have
Q99: The equilibrium constant,K,can be calculated from<br>A)E°.<br>B)E.<br>C)either E°
Q100: Which is the best acid to use
Q105: Which of the following changes in reaction
Q178: What is the K<sub>a</sub> of the amino
Q221: Dihydrogen phosphate H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,has an acid dissociation constant