The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 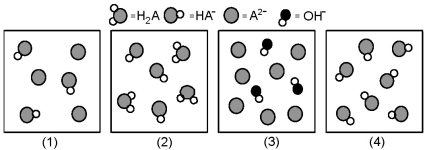
-Which picture represents the system beyond the second equivalence point?
Definitions:
Q9: Which of the following statements must be
Q22: A reaction has a rate constant,k =
Q90: For the reaction: N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)⇌ 2
Q100: For which one of the following reactions
Q112: Which picture represents the system beyond the
Q132: The cell reaction for a lead storage
Q147: A weak base ionizes in water as
Q150: Which point a-d represents pK<sub>a2</sub>?<br>A)point a<br>B)point b<br>C)point
Q166: What is the value for K<sub>c</sub> for
Q185: What is the hydronium ion concentration and