The following pictures represent solutions of AgCl,which may also contain ions other than Ag+ and Cl- which are not shown.Gray spheres represent Ag+ ions and dotted spheres represent Cl- ions. 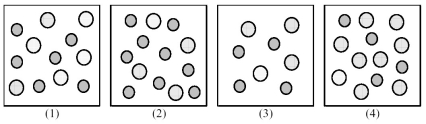
-If solution (1) is a saturated solution of AgCl,which of solutions (1) -(4) represents the solution after a small amount of HNO3 is added and equilibrium is restored?
Definitions:
Q26: Identify the conjugate acid/base pairs present in
Q33: Which of the following is true?<br>A)As a
Q40: What are the signs (+ or -)of
Q53: The standard molar entropy for Br<sub>2</sub>(g)is 245.46
Q129: According to the third law of thermodynamics,<br>A)energy
Q138: Sulfurous acid,H<sub>2</sub>SO<sub>3</sub> has acid dissociation constants K<sub>a1</sub>
Q144: What is the pH of a 0.040
Q155: Which picture (2)-(4)represents the equilibrium mixture when
Q183: Which picture represents the equilibrium state of
Q200: The following cell has a potential of