The following pictures represent solutions of CaCO3,which may also contain ions other than Ca2+ and CO32- which are not shown.Gray spheres represent Ca2+ ions and unshaded spheres represent CO32- ions. 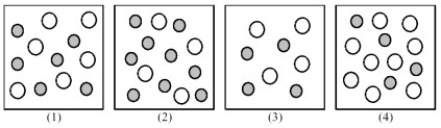
-If solution (1) is a saturated solution of CaCO3,which of solutions (1) -(4) represents the solution after a small amount of HNO3 is added and equilibrium is restored?
Definitions:
Management by Exception
A management strategy where only significant deviations from expected outcomes are addressed, focusing leaders' attention on the most important issues.
Responsibility Accounting
An accounting system that measures the results of each responsibility center and compares those results with some measure of expected or budgeted outcome.
Responsibility Reporting System
The preparation of reports for each level of responsibility in the company’s organization chart.
Static Budget
A fixed budget that does not change or adapt based on any variations in a company's activity levels or performance.
Q18: Which picture represents a neutral salt?<br>A)(1)<br>B)(2)<br>C)(3)<br>D)None of
Q22: If ΔG° is negative for a reaction,<br>A)K
Q35: For a particular cell based on the
Q45: The standard reduction potential E° = -0.45
Q69: The decomposition of ammonia is: 2 NH<sub>3</sub>(g)⇌
Q92: From the following chemical reactions determine the
Q103: Beta decay of <sup>24</sup>Na produces a beta
Q139: Which of the following is zero at
Q169: A solution may contain the following ions
Q193: The equilibrium constant for the reaction below