The following pictures represent solutions of CaCO3,which may also contain ions other than Ca2+ and CO32- which are not shown.Gray spheres represent Ca2+ ions and unshaded spheres represent CO32- ions. 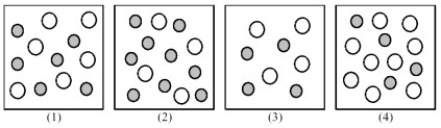
-If solution (1) is a saturated solution of CaCO3,which of solutions (1) -(4) represents the solution after a small amount of Ca(NO3) 2 is added and equilibrium is restored?
Definitions:
Q3: What is the scandium ion concentration for
Q56: What is the pH of a 2.4
Q75: For the galvanic cell Pt(s)∣ Sn<sup>2+</sup>(aq),Sn<sup>4+</sup>(aq)∣∣ Cd<sup>2+</sup>(aq)∣
Q84: The reaction below virtually goes to completion
Q85: What is the pH of a buffered
Q92: The pink and blue species below form
Q108: What is true when the following equation
Q131: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q136: For the reaction: 4 HCl(g)+ O<sub>2</sub>(g)⇌ 2
Q158: A crude type of disappearing ink is