What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of 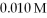 NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
Definitions:
Decrease Of
Refers to the reduction in the amount, quantity, or extent of something.
Percent Less
A term used to indicate a reduction or discount applied to a price or value, expressed as a percentage of the original amount.
Percent More
A comparative measure indicating how much higher one quantity is than another, expressed as a percentage.
Percent Change
The measure of the degree of change of a quantity, expressed as a percentage.
Q48: What is the identity of M in
Q48: In which of the following solutions would
Q52: Four heavy elements (A, B, C, and
Q98: Addition of 0.0125 mol KOH to 150
Q98: Determine the acid dissociation constant for a
Q124: Which of the following Br∅nsted-Lowry acids does
Q129: What is the second stepwise equilibrium constant
Q131: Sodium hypochlorite,NaOCl,is the active ingredient in household
Q135: The total binding energies for <sup>3</sup>He,<sup>4</sup>He,and <sup>6</sup>He
Q166: What is the pH at the first