The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 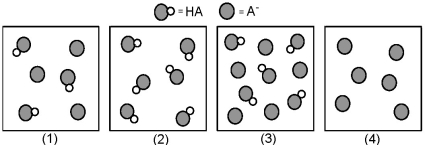
-Which solution has the largest percent dissociation of HA?
Definitions:
Annual Net Cash Flows
The amount of cash a company generates after all its expenditures, both operational and capital in nature, have been paid off within a fiscal year.
Average Rate of Return
A financial metric used to calculate the average annual return on an investment or project over its lifetime.
Total Income
The sum of all earnings or revenues generated by an individual or entity within a specific period.
Residual Value
The estimated value that an asset will realize upon its sale at the end of its useful life.
Q18: What is the pH of a solution
Q30: The pH of 0.255 M HCN is
Q41: In which of the following solutions would
Q57: The nuclear decay process that involves the
Q71: Ag<sup>+</sup>(aq)+ e<sup>-</sup> → Ag(s)E° = +0.800 V
Q101: Consider the following cell: Pt(s)∣ H<sub>2</sub>(g,p<sub>1</sub>)∣ H<sup>+</sup>(aq,pH<sub>A</sub>)∣∣
Q102: Consider the reaction: N<sub>2</sub>(g)+ 3 F<sub>2</sub>(g)→ 2
Q158: Based on the balanced chemical equation shown
Q187: A reaction is second order in NO
Q202: Aluminum requires relatively little protection from corrosion