The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 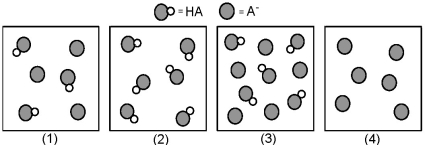
-Which solution has the lowest pH?
Definitions:
Third-Degree Burns
Severe burns that destroy both the epidermis and dermis layers of skin, potentially affecting underlying tissues, requiring medical intervention.
Peripheral Neuropathy
A condition resulting from damage to the peripheral nerves, characterized by pain, numbness, and muscle weakness in the limbs.
Type 2 Diabetes
A chronic condition characterized by high blood sugar levels due to insulin resistance or a lack of insulin production.
Therapeutic Response
The body's reaction to a treatment, indicating the effectiveness of an intervention.
Q1: For the reaction shown below the equilibrium
Q4: Given the half-cell potentials below,calculate the cell
Q10: For a galvanic cell,the cathode has a
Q47: What is the pH of a solution
Q54: Is the cell shown above a galvanic
Q68: Gaseous hydrogen bromide decomposes at elevated temperatures
Q101: For the reaction N<sub>2</sub>(g)+2 O<sub>2</sub>(g)→ 2 NO<sub>2</sub>(g),ΔH°
Q128: When suspected drunk drivers are tested with
Q133: What is the hydroxide ion concentration of
Q165: Which one of the following salts,when dissolved