The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 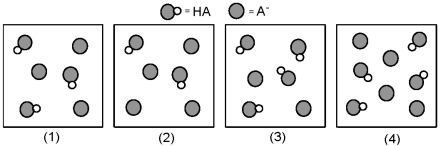
-Which of these solutions are buffers?
Definitions:
Compensate
To make up for something undesired by exerting an effort or offering something in return.
Cultivating
The process of trying to acquire or develop a quality, sentiment, or skill.
Multidimensional
Having or relating to multiple aspects, factors, or components.
Socioemotional Change
Variations in emotional and social behavior that occur over the lifespan, influenced by a combination of individual, contextual, and cultural factors.
Q2: Which of the following processes are spontaneous?
Q28: According to the balanced equation shown below,1.00
Q34: What is the strongest acid among the
Q67: ΔS° = -198.7 J/K for the reaction
Q124: Which of the following Br∅nsted-Lowry acids does
Q126: Using the following standard reduction potentials, Fe<sup>3+</sup>(aq)+
Q135: If solution (1)is a saturated solution of
Q137: The reaction 2 H<sub>2</sub>O(g)+ CO<sub>2</sub>(g)→ CH<sub>4</sub>(g)+ 2
Q152: The standard cell potential for the following
Q193: The equilibrium constant for the reaction below