The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 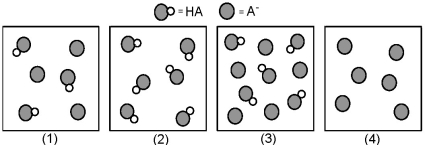
-Which solution has the greatest buffer capacity?
Definitions:
Adjusted Balance
A method of calculating account balances that takes into account all debits and credits, including pending transactions, at the end of a period.
Depreciation Expense
The systematic allocation of the cost of a tangible asset over its useful life, reflecting the decrease in value of the asset over time.
Useful Life
The period of time over which an asset is expected to be usable by an organization, affecting its depreciation or amortization schedules.
Depreciation Expense
The allocated portion of the cost of an asset, spread out over its useful life, representing wear and tear, obsolescence, or a decline in value.
Q10: What is the pH at the equivalence
Q57: Which has the lowest entropy?<br>A)CH<sub>3</sub>OH(s,-26°C)<br>B)CH<sub>3</sub>OH(s,-13°C)<br>C)CH<sub>3</sub>OH(l,16°C)<br>D)CH<sub>3</sub>OH(l,30°C)
Q81: The number of nucleons in a <img
Q82: If K<sub>c</sub> = 0.600,and K<sub>p</sub> = 359
Q99: If Q increases,<br>A)ΔG increases and the reaction
Q110: A solution with a hydroxide ion concentration
Q142: Arrange the acids in order of increasing
Q145: Calculate the concentration of bicarbonate ion,HCO<sub>3</sub><sup>-</sup>,in a
Q176: A solution may contain the following ions
Q186: What is the hydronium ion concentration in